Nov 26, 2024
Changes in intestinal mucosal barrier function in obese mice
- xiaolin Gao1,
- Mingli Guan1,
- Qiuju Li1,
- Ruizhen Jia1,
- Jianjun Deng1
- 1West China Second Hospital
- xiaolin Gao: 1
- Mingli Guan: 2
- Qiuju Li: 3
- Ruizhen Jia: 4
- Jianjun Deng: 5

Protocol Citation: xiaolin Gao, Mingli Guan, Qiuju Li, Ruizhen Jia, Jianjun Deng 2024. Changes in intestinal mucosal barrier function in obese mice. protocols.io https://dx.doi.org/10.17504/protocols.io.bp2l6dbw5vqe/v1
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
We use this protocol and it's working
Created: November 25, 2024
Last Modified: November 26, 2024
Protocol Integer ID: 112772
Keywords: Intestinal mucosal barrier Function, Obesity, Mice
Disclaimer
The authors have no financial relationships relevant to this article to disclose.
Abstract
Introduction Few reports have described changes in other functions of the intestinal mucosal barrier in obese patients worldwide. Objectives To observe the changes in intestinal mucosal barrier function in obese mice. Methods Fifty healthy male C57BL/6J mice were randomly divided into a high-fat diet (HFD)-induced obese group and a control group (CON), with 25 mice in each group. Plasma diamine oxidase (DAO), D-lactate and endotoxin levels were detected via the enzyme-labelled colorimetric method. Characteristics of intestinal flora in faeces were detected via Illumina HiSeq 16S rDNA high-throughput sequencing technology. The morphological characteristics of the ileal mucosa were observed via optical microscopy. The data were analysed via bioinformatics and statistics. Results The plasma levels of DAO, D-lactate, and
endotoxin levels in the HFD group were significantly higher than those in the CON group at week 10(P<0.05). The plasma DAO, D-lactate, and endotoxin levels in the HFD group were significantly greater than in the CON group (P<0.05). The CON group contained more intestinal microbiota phyla and genera than the HFD group; the differences between the two groups were significant(FDR≤0.05, P≤0.05). The ileal mucosa of the HFD group exhibited more epithelial shedding at the intestinal villus tip, and the intestinal glands were more abundant. Conclusion This study is expected to provide a basic experimental reference for further research on the mechanism, prevention, and treatment of obesity.
Image Attribution
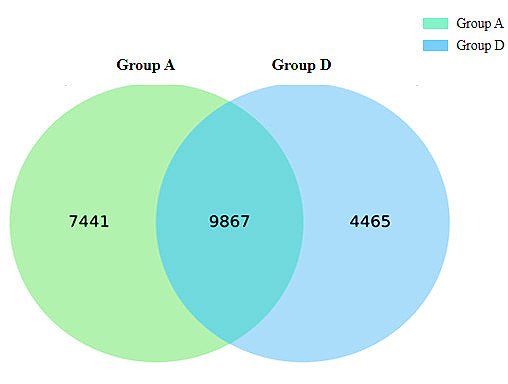
Figure
1 OTU Venn Figure
Figure
2 Box-plot of α diversity of the obesity group and the control group
Figure
3 PCoA analysis of the obesity group and the control group based on OTU
abundance
Figure
4 Histogram of the abundance distribution of phylum and genus of intestinal
microbiota(Top15)
Figure
5 Results of HE staining of mouse ileal mucosal sections (Under an optical
microscope, 40Times)
Guidelines
To ensure the accuracy of the results, precise declutter can be performed to remove sequences containing ambiguous bases, single-base high-repeating regions, and excessively short sequences. The parameters for precise decontamination are: the sequence containing N bases is removed, and the sequence with a base mass fraction Q20 of at least 75% is retained. At the same time, the chimeric sequences were detected and removed by UCHIME.
After the sequencing data were preprocessed to generate high-quality sequences, Vsearch[5] software was used to classify sequences into multiple OTUs according to their similarity. The argument that sequence similarity is greater than or equal to 97% is classified as an OTU cell.
The QIIME [6] package was used to pick out the representative sequences of each OTU and annotate all the representative sequences against the database. Silva (version138) database was used for comparison, and the RDP classifier [7] software was used for species comparison annotations, and the annotation results with confidence intervals greater than 0.7 were retained.0 µL 0 µL 0 µL
Materials
Animals
Healthy C57BL/6J SPF-grade mice (male; 6 weeks old; body weight, 23–28g) with the diet purchased from Chengdu Quan Xi Biotechnology Co., Ltd. The mice were adaptively fed for 1 week before the experiment. Humidity was controlled within 50% to 70%, and the temperature was maintained between 20 to 24 °C, with a 12-hour light/dark cycle. The animals had free access to food and water.
The mice were randomly divided into 2 groups using the computer method, namely, the HFD group and the control group, with 25 mice in each group. The living environments of the two groups were identical, and the experimental observation period was 10 weeks. The HFD group was fed a high-fat diet (fat 60.0%, protein 19.4%, carbohydrate 20.6%; calories 5.0 kcal/g), and the CON group was fed a normal diet (fat 10.0%, protein 19.0%, carbohydrate 71.0%; calories 3.6 kcal/g).Two grams of fresh feces were collected at fixed time points in week 1 and week 10 respectively and stored in the -80 °C refrigerator. All the mice were euthanized by pentobarbital at week 10. Abdominal white adipose and ileum tissues were collected for examination. Blood samples were collected, centrifuged, and stored in a -20 °C freezer until analysis.
Safety warnings
This protocol contains confidential information and/or information protected by intellectual property rights for the exclusive attention of the intended viewers only. Please avoid printing, making screenshots and distributing.
Ethics statement
This study was approved by the Ethical Committee on Animal Experimentation of the Ethics Committee of West China Second Hospital, Sichuan University (NO.2020008). All methods were carried out in accordance with relevant guidelines and regulations.
16S rRNA amplicon sequencing
16S rRNA amplicon sequencing
16S rRNA amplicon sequencing analysis process
DNA extraction and amplification
Library construction and sequencing
Bioinformatic analysis
16S rRNA amplicon sequencing analysis process
Protocol references
[1] Bajaj SS, Jain B, Kyle TK, et al.Overcoming
congressional inertia on obesity requires better literacy in obesity
science.OBESITY,2002, 30(4):799-801.
[2] Tahrani AA, Morton J.Benefits of weight
loss of 10% or more in patients with overweight or obesity: A review.OBESITY,
2022, 30(4):802-840.
[3] Serrano-Fuentes N, Rogers Anne, Portillo MC. Beyond
individual responsibility: Exploring lay understandings of the contribution of
environments on personal trajectories of obesity. PLoS One, 2024,
19(5):e0302927.
[4] Gao X, Jia R, Xie L, et al. A study
of the correlation between obesity and intestinal flora in school-age children.
Sci Rep, 2018, 8(1):14511.
[5] Le Roy T, Moens de Hase E, Van Hul M, et
al. Dysosmobacter welbionis is a newly isolated human commensal bacterium
preventing diet-induced obesity and metabolic disorders in mice.GUT,
2022,71(3):534-543.
[6] Li
QJ, Gao XL, Jia RZ, et al. Establishment of a novel obesity mouse model: the
induction of intestinal microbiota dysbiosis. Sci Rep, 2024, 14(1):15950.
[7] Huang L, He F, Wu B. Mechanism of effects of nickel or
nickel compounds on intestinal mucosal barrier. CHEMOSPHERE, 2022, 305:135429.
[8] An J, Liu Y, Wang Y, et al. The Role of
Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front
Immunol, 2022,13: 871713.
[9] Bliesner A, Eccles-Smith J, Bates C, et al.
Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals
with Obesity: A Systematic Review.Nutrients. 2022-05-06; 14(9).
[10] Yang J, Xiong P, Bai L, et al. The Association
of Altered Gut Microbiota and Intestinal Mucosal Barrier Integrity in Mice With
Heroin Dependence.Front Nutr, 2021, 8:765414.
[11] Gao X, Miao R, Tao Y, et al. Effect of
Montmorillonite powder on intestinal mucosal barrier in children with abdominal
Henoch-Schonlein purpura: A randomized controlled study. MEDICINE, 2018,
97(39):e12577.
[12] Chen S, Xiu G, Zhou J, et al. Role of high
mobility group box 1 in intestinal mucosal barrier injury in rat with sepsis
induced by endotoxin. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 2020,
32(7):803-807.
[13] Dieryck I, De Backere J, Paeshuyse J. Effect of
hatching system and prophylactic antibiotic use on serum levels of intestinal
health biomarker diamine oxidase in broilers at an early age. ANIMAL, 2022,
16(4):100493.
[14] Zhang X, Liu D, Wang Y, et al. Clinical
significance on serum intestinal fatty acid binding protein and D-lactic acid
levels in early intestinal injury of patients with sepsis.Zhonghua Wei Zhong
Bing Ji Jiu Yi Xue, 2019,31(5):545-550.
[15] Huang J, Guan B, Lin L, et al. Improvement of
intestinal barrier function, gut microbiota, and metabolic endotoxemia in type
2 diabetes rats by curcumin.BIOENGINEERED, 2021, 12(2):11947-11958.
Acknowledgements
The authors would like to thank all study subjects and the staff involved in this study.